Endoscopic Management of Post-Sphincterotomy Bleeding: Eight Hemostatic Tips
By Klaus Mönkemüller, MD, PhD, FASGE, FJGES
Professor of Medicine, Virginia Tech Carilion School of Medicine, Virginia, USA, Honorary. Professor, Universidad de La República, Montevideo, Uruguay, Honorary Professor, UEES, Guayaquil, Ecuador

Endoscopic-Management-of-Post-Sphincterotomy-Bleeding-Eight-Hemostatic-Tips.pdf
1.75 MB • PDF File
Post-sphincterotomy bleeding is a significant concern in endoscopic retrograde cholangiopancreatography (ERCP) procedures (1). While literature reports suggest that bleeding occurs in less than 1% of ERCPs involving biliary or pancreatic sphincterotomy, clinical experience often indicates a higher frequency, as currently more patients are on chronic anticoagulation. This discrepancy underscores the importance of being well-prepared to manage such complications.
The ampulla of Vater and its surrounding structures have an incredibly rich blood supply, which contributes to the risk of bleeding during and after sphincterotomy (2, 3). This vascular network includes branches from the gastroduodenal artery, inferior pancreaticoduodenal artery, anterior and posterior pancreaticoduodenal arcades, and the anterior superior pancreaticoduodenal artery, among others (2, 3). Understanding this complex vascular anatomy is crucial for effective management of post-sphincterotomy bleeding.
Understanding the Anatomy
To effectively manage post-sphincterotomy bleeding, it’s essential to have a thorough understanding of the relevant anatomy. The bile duct and sphincter area have a figure-of-eight configuration, not the simple circular shape often depicted in medical textbooks. This figure-of-eight shape influences the direction of cuts made during sphincterotomy procedures.
For biliary sphincterotomy, the cut is typically made towards the 11 o’clock position, while for pancreatic duct sphincterotomy, the cut is directed towards the 1 o’clock position. These directions are chosen to minimize the risk of cutting through major blood vessels, as histological studies have shown that the largest concentration of blood vessels is at the 9 and 3 o’clock positions.
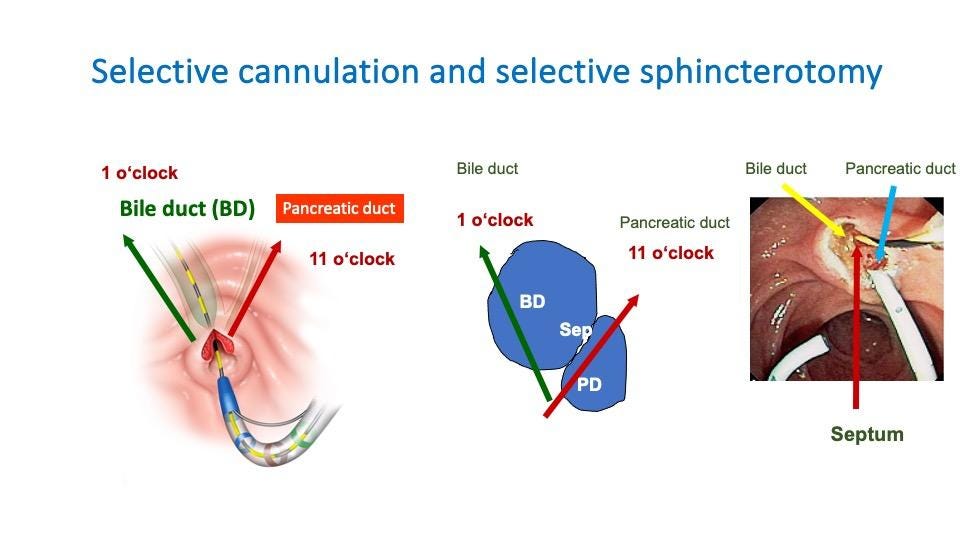
Figure 1. Cannulation and sphincterotomy directions. The septum between the biliary and pancreatic ducts is another crucial anatomical feature to consider. Understanding its position can aid in selective cannulation and help avoid unintended damage during sphincterotomy.
Challenges in Hemostasis
Several factors can complicate hemostasis in post-sphincterotomy bleeding (Figure 2):
Awkward scope positions: The duodenoscope may be in a challenging position, making it difficult to manipulate instruments effectively.
Surgical altered anatomy: Previous surgeries, such as Billroth II procedures, can significantly alter the anatomy, making access and visualization more challenging.
Abnormal papilla appearance or location: The papilla may appear unusual, be hanging loosely, or be located inside a diverticulum or on its edge. These variations can make it harder to identify and treat the source of bleeding.
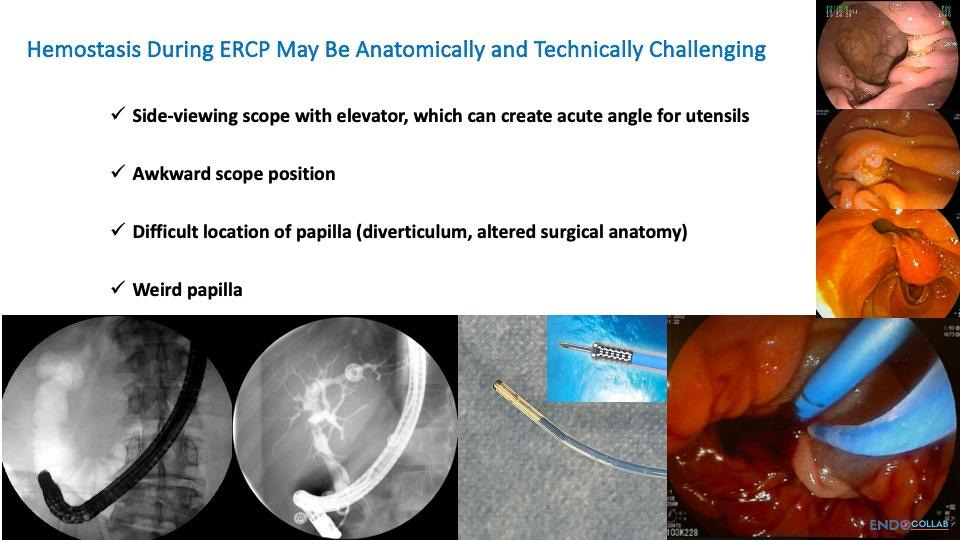
Figure 2. Challenges for hemostasis during ERCP.
Given these potential challenges, it’s crucial to have a variety of hemostatic techniques at your disposal. Let’s explore eight effective tricks for managing post-sphincterotomy bleeding.
Trick 1: The Manta Needle
The Manta needle is an invaluable tool for managing post-sphincterotomy bleeding (Figure 3). Its key features include:
A thin outer diameter (approximately 2mm), allowing easy passage through the working channel of any endoscope, especially duodenoscopes.
A reinforced tip that enables smooth advancement through the endoscope, even when the elevator is in the up position. Other needles (with the exception of the Carr-Locke needle) usually puncture through the sheet when closing the elevator (“up position”).
Resistance to breaking through the plastic sheath, a common problem with other needles.
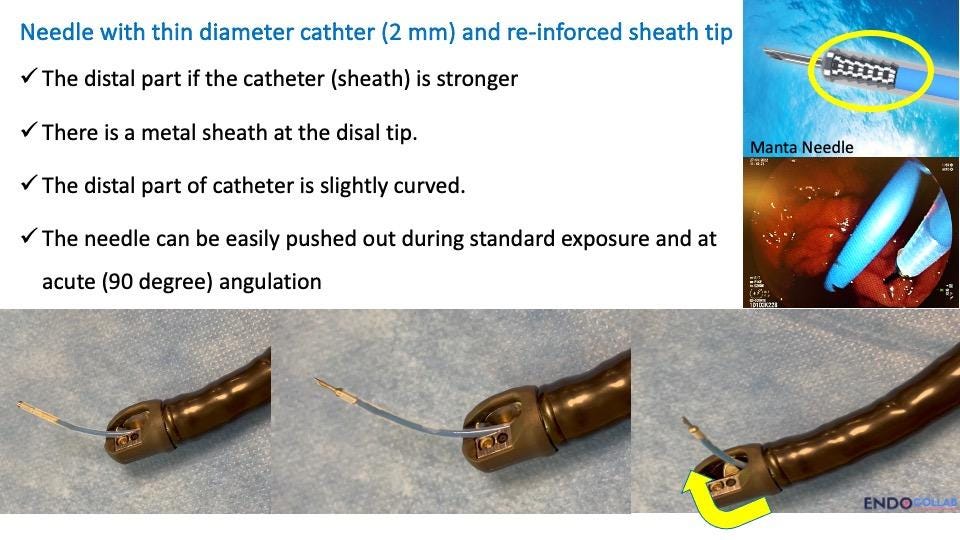
Figure 3. Thin needle with reinforced tip. The needle can be easily pushed out with elevator in the “up” position (curved yellow arrow).
To use the Manta needle effectively:
Advance the needle towards the bleeding area around the biliary or pancreatic sphincter.
Inject a solution of epinephrine and saline. The epinephrine provides immediate vasoconstriction, while the saline creates tamponade through local tissue edema.
Inject in multiple areas around the bleeding site to ensure comprehensive coverage.
Remember, while epinephrine injection can provide rapid hemostasis, it’s often temporary. Additional hemostatic measures are usually necessary for definitive treatment.
Trick 2: Clips for Hemostasis
Endoscopic clips are another essential tool for managing post-sphincterotomy bleeding. When using clips, consider the following:
Clip design: Pay attention to both the arms and stems of the clips (Figure 4). Clips with shorter stems are generally more practical for papillary bleeding, as they’re less likely to get caught on the elevator of the duodenoscope.
Clip exposure: When using a duodenoscope, keep the plastic sheath on the clip in place. This helps prevent premature deployment and allows for better control.
Clip manipulation: After exposing the clip, open it fully. You can then use the duodenoscope’s controls to bow the clip up and down, allowing for precise positioning (Figure 5).
Clip size: For papillary bleeding, 11mm clips are usually sufficient, as the goal is to close individual vessels rather than large defects.
Figure 4 shows various types of commercially available clips.
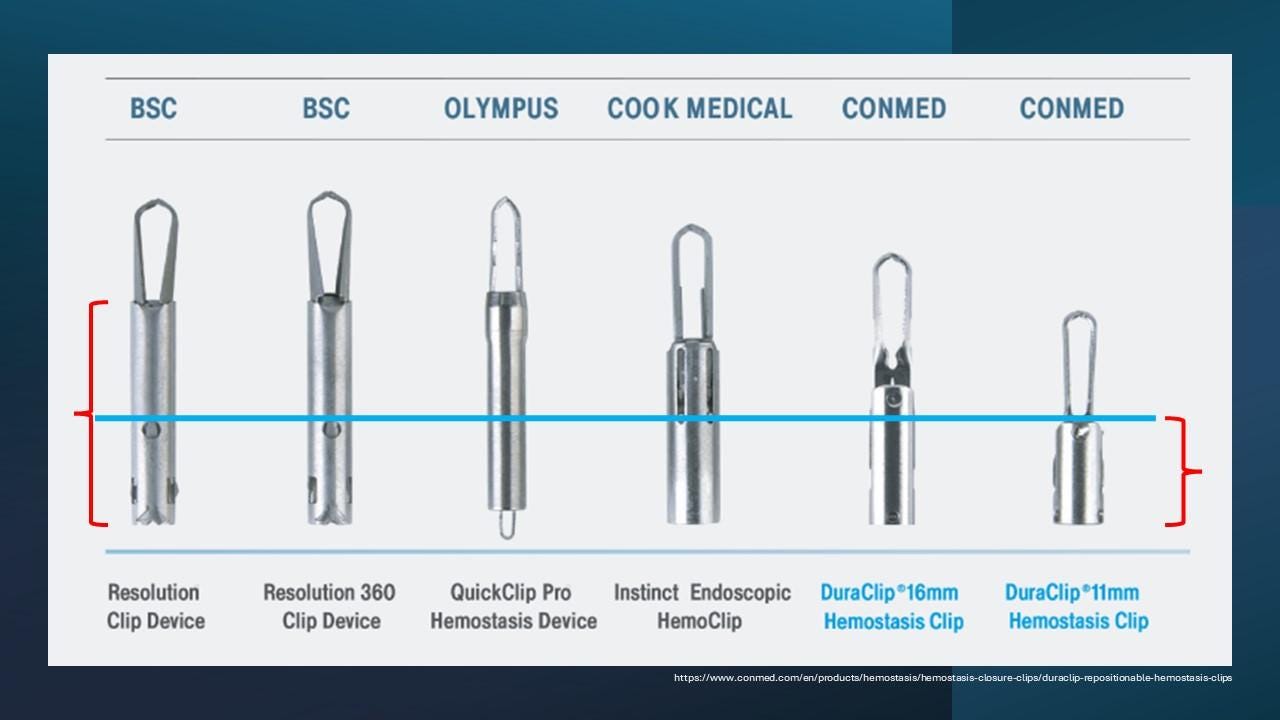
Figure 4. Various types of commercially available clips.

Figure 5. How to choose a clip for ERCP scope. Using clips with shorter stem may improve manipulation while elevator is in the “up” position.
When applying clips:
Identify the bleeding vessel or area.
Position the clip perpendicular to the vessel if possible.
Apply the clip, ensuring it grasps enough tissue to provide effective hemostasis.
Be prepared to apply multiple clips if necessary.
Trick 3: Caps in Endoscopy
Endoscopic caps can be incredibly useful for managing post-sphincterotomy bleeding, especially when using a forward-viewing endoscope (4). Key points about using caps include:
Cap selection: Choose barrel-shaped caps with side openings to allow for fluid drainage and maintain visibility.
Improved visualization: Caps help maintain a clear field of view by creating distance between the tip of the endoscope and the tissue.
Clip assistance: When using clips, the cap can help keep the clip open as you approach the target area, allowing for more precise placement.
Compression: The cap can be used to apply direct pressure to the bleeding site, providing temporary hemostasis while preparing other interventions.
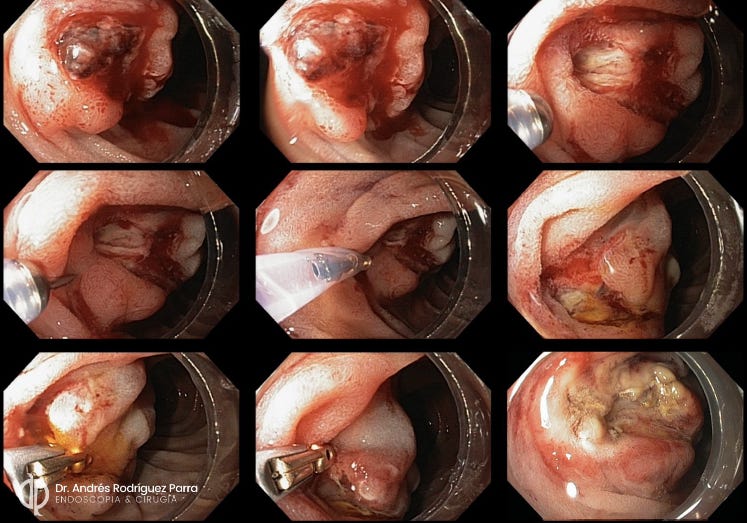
Figure 6. Cap-assisted hemostasis for post-sphincterotomy bleeding. Source: Dr. Andrés Rodríguez-Parra, Colombia.
To use a cap effectively:
Attach the appropriate cap to your endoscope.
Use the cap to locate the papilla and identify the bleeding site.
If using clips, pre-open the clip within the cap before approaching the target area.
Apply gentle pressure with the cap to temporarily control bleeding and improve visualization.
Deploy your chosen hemostatic method (e.g., clip, injection) with the assistance of the cap.
Trick 4: Fully Covered Self-Expanding Metal Stents
Fully covered self-expanding metal stents (FCSEMS) can be an excellent option for managing difficult post-sphincterotomy bleeding (Figure 7). They work by applying consistent pressure to the entire circumference of the sphincterotomy site, effectively tamponading any bleeding vessels.
Figure 7. Fully-covered self-expanding metal stents for post-sphicnterotomy bleeding.
Key points for using FCSEMS:
Always have a FCSEMS available when performing ERCP procedures.
Choose an appropriate size based on the bile duct diameter and the extent of the sphincterotomy.
FCSEMS can be particularly useful in cases of altered anatomy, such as Billroth II, where other hemostatic techniques may be challenging.
To deploy a FCSEMS for hemostasis:
Confirm the source of bleeding and assess the bile duct size.
Choose an appropriately sized FCSEMS (based on diameter of bile duct).
Deploy the stent across the sphincterotomy site, ensuring it covers the entire area of concern.
Confirm proper placement with fluoroscopy and direct visualization if possible.
Plan for stent removal in 1-2 weeks, once hemostasis is assured and the risk of recurrent bleeding has decreased.
Trick 5: Coagrasper and Ensure Coagulation Forceps
The Coagrasper (Olympus) and Ensure Coagulation Forceps (Microtech) are specialized devices designed for precise coagulation of bleeding vessels (Figure 8). These tools are particularly useful for targeting specific bleeding points that may be difficult to address with other methods (5).
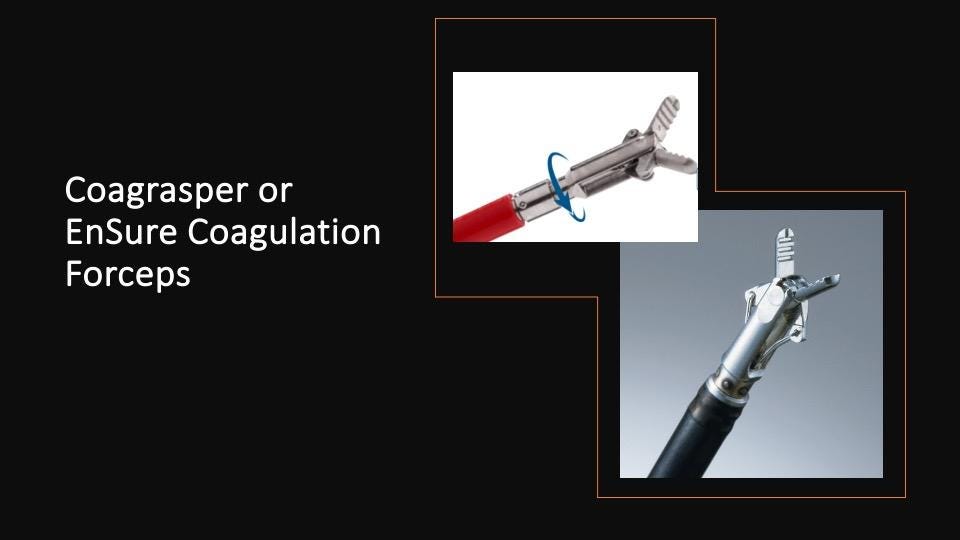
Figure 8. Hemostatic forceps.
Key features and usage tips:
These devices use monopolar electrocoagulation to seal bleeding vessels.
They are most effective for vessels less than 2mm in diameter.
When using an ERBE electrosurgical unit, a setting of soft coagulation with effect 5 is typically recommended.
To use these devices effectively:
Identify the specific bleeding vessel or point.
Open the forceps and approach the target area.
Grasp the bleeding point with the forceps.
Apply coagulation current for 1-2 seconds.
Release the tissue and assess for hemostasis.
Repeat if necessary, being cautious not to apply excessive coagulation to any one area.
Trick 6: Self-Assembling Gels (e.g., Purastat)
Self-assembling gels, such as Purastat, are newer additions to the hemostatic arsenal. These products can serve two primary functions in managing post-sphincterotomy bleeding (6, 7):
Vessel identification: The gel can help localize the source of bleeding by adhering to the bleeding site and making it more visible.
Direct hemostasis: In some cases, the gel itself can provide effective hemostasis by forming a barrier over the bleeding site.
To use self-assembling gels:
Prepare the gel according to the manufacturer’s instructions.
Apply the gel to the area of suspected bleeding using the provided catheter.
Observe the area to identify any points where the gel is displaced by active bleeding.
If bleeding continues, use the gel-highlighted areas to guide more targeted hemostatic interventions.
In cases of mild oozing, the gel may be sufficient to achieve hemostasis on its own.
Trick 7: Balloon Dilation for Compression Hemostasis
Balloon dilation can be an effective method for achieving hemostasis through direct compression. This technique is particularly useful when the bleeding source is diffuse or when other methods have failed.
Figure 9. Balloon dilation.
Key points for balloon compression:
Use esophageal balloons rather than biliary balloons for this purpose. Esophageal balloons provide better compression and are more durable.
Choose the balloon size based on the diameter of the bile duct. For a 12-15mm bile duct, use a 12-15mm esophageal balloon.
To perform balloon compression:
Select an appropriately sized esophageal balloon.
Position the deflated balloon across the sphincterotomy site.
Slowly inflate the balloon to the desired diameter.
Maintain inflation for 3-5 minutes.
Slowly deflate the balloon and assess for ongoing bleeding.
Repeat if necessary, being cautious not to overinflate or maintain inflation for too long, which could lead to ischemic injury.
Trick 8: Interventional Radiology
When endoscopic methods fail to achieve hemostasis, interventional radiology can be a life-saving option. It’s important to consider this option early in cases of severe or refractory bleeding.
Key points about interventional radiology for post-sphincterotomy bleeding:
It’s generally preferred over surgery due to its less invasive nature.
Selective embolization can target specific bleeding vessels while preserving surrounding tissue.
Multiple attempts may be necessary, as there can be more than one bleeding source.
When to consider interventional radiology:
Severe bleeding that doesn’t respond to initial endoscopic measures.
Recurrent bleeding after apparent initial hemostasis.
Hemodynamic instability despite aggressive resuscitation and endoscopic attempts at hemostasis.
The interventional radiologist will typically perform an angiogram to identify the bleeding vessels and then selectively embolize them. This procedure may need to be repeated if there are multiple bleeding sources or if rebleeding occurs.
Conclusion
Managing post-sphincterotomy bleeding requires a multi-faceted approach and a well-stocked arsenal of hemostatic techniques. By mastering these eight tricks – the Manta needle, endoscopic clips, caps, fully covered self-expanding metal stents, coagulation forceps, self-assembling gels, balloon compression, and interventional radiology – endoscopists can effectively manage most cases of post-sphincterotomy bleeding.
Remember that each case is unique, and often a combination of these techniques may be necessary to achieve definitive hemostasis. Always be prepared with multiple options, and don’t hesitate to escalate to more advanced techniques or seek assistance from colleagues in interventional radiology when needed. With practice and experience, you’ll develop the skills to confidently handle this challenging complication of ERCP procedures.
References:
Wilcox CM, Canakis J, Mönkemüller KE, Bondora AW, Geels W. Patterns of bleeding after endoscopic sphincterotomy, the subsequent risk of bleeding, and the role of epinephrine injection. Am J Gastroenterol. 2004 Feb;99(2):244-8. doi: 10.1111/j.1572-0241.2004.04058.x. PMID: 15046211.
Purvis NS, Mirjalili SA, Stringer MD. The mucosal folds at the pancreaticobiliary junction. Surg Radiol Anat. 2013 Dec;35(10):943-50. doi: 10.1007/s00276-013-1128-y. Epub 2013 May 5. PMID: 23645171.
Mirjalili SA, Stringer MD. The arterial supply of the major duodenal papilla and its relevance to endoscopic sphincterotomy. Endoscopy. 2011 Apr;43(4):307-11. doi: 10.1055/s-0030-1256229. Epub 2011 Mar 31. PMID: 21455871.
Chon HK, Kim TH. Endoclip therapy of post-sphincterotomy bleeding using a transparent cap-fitted forward-viewing gastroscope. Surg Endosc. 2017 Jul;31(7):2783-2788. doi: 10.1007/s00464-016-5287-x. Epub 2016 Oct 19. PMID: 27761747.
Bapaye JA, Wasserman RD, Mönkemüller K, Kesar V, Kesar V. Application of hemostatic forceps to treat post-sphincterotomy bleeding near the pancreatic duct opening. Endoscopy. 2024 Dec;56(S 01):E630-E631. doi: 10.1055/a-2353-5910. Epub 2024 Jul 26. PMID: 39059447; PMCID: PMC11281839.
Ogura T, Uba Y, Yamamura M, Nishioka N, Nishikawa H. Endoscopic hemostasis using self-expandable metal stent combined with PuraStatⓇ for patient with high risk of post-endoscopic sphincterotomy bleeding (with video). Hepatobiliary Pancreat Dis Int. 2024 Feb;23(1):94-96. doi: 10.1016/j.hbpd.2023.02.001. Epub 2023 Feb 4. PMID: 36754705.
Ishida Y, Tsuchiya N, Koga T, Kitaguchi T, Matsumoto K, Kuno N, Funakoshi S, Ishibashi H, Ashizuka S, Hirai F. A novel self-assembling peptide hemostatic gel as an option for initial hemostasis in endoscopic sphincterotomy-related hemorrhage: a case series. Clin J Gastroenterol. 2022 Dec;15(6):1210-1215. doi: 10.1007/s12328-022-01702-9. Epub 2022 Sep 19. PMID: 36121586.
Disclosures: KM- no COI with any products, devices or companies mentioned in this article.
Next Steps:
Join EndoCollab: EndoCollab is your go-to platform for advancing your skills in gastroenterology and endoscopy. Gain access to exclusive content from top practitioners to enhance your practice.
Membership Features:
Video lessons on essential and advanced techniques.
Expert-led classes and practical tips.
Community-driven insights and peer discussions.
Join 1,200+ healthcare professionals taking their expertise to the next level.
Join the WhatsApp Group: An exclusive WhatsApp group for gastroenterologists and endoscopists. Connect with leading experts, discuss challenging cases, and stay ahead with the latest in clinical research and best practices. This group is your gateway to a vibrant community of like-minded professionals committed to advancing GI care.
Join the WhatsApp group today

