Superior Mesenteric Artery Syndrome
Jonathan Rozenberg, DO, MPH; Jay A. Bapaye, MD; Klaus Mönkemüller, MD, PhD, FASGE, FJGES; Adil S. Mir, MD, FACP
Institution: Virginia Tech Carilion School of Medicine, Roanoke, VA, USA
Superior mesenteric artery syndrome (SMAS) depicts the rare phenomena of the third (or horizontal) portion of the duodenum being compressed between the superior mesenteric artery (SMA) and abdominal aorta (AA), resulting in proximal small bowel obstruction (1-4). SMAS was first described as a case report by Carl von Rokitansky in 1842, and later expanded upon–with regards to pathophysiology and diagnostic findings–by David Wilkie in 1927. SMAS has also been referred to as Wilkie’s syndrome or aorto-mesenteric compass syndrome (1,3,4).
Typically, a mesenteric fat pad separates the SMA and AA thereby preventing constriction of the third portion of the duodenum by these structures. Thus, factors which impact the anatomical balance of these structures lead to a decrease in SMA-AA angle and distance–ultimately allowing for duodenal compression. One example would be significant weight loss as this decreases the proportion of mesenteric fat present. Numerous etiologies can cause this such as: hypermetabolic state (e.g., drug-induced, burn-related trauma), diet disorders (e.g., anorexia nervosa), malabsorptive disease (e.g., celiac disease, crohn’s disease), or cachexia-inducing conditions (e.g., malignancy, tuberculosis, AIDS) (1-4).
There are conflicting reports with regards to an accurate prevalence of SMAS; however, the reported incidence has been approximated, via radiographic studies, to 0.013%-0.780% (1), with other reports narrowing this range to 0.1%-0.3% (3). A recent SMAS review by Oka et al (2023) reported a median age of 23 years old with an interquartile range of 16-39 but did acknowledge the recent literature reporting an increasing number of SMAS cases in the elderly. As etiology of SMAS can vary greatly between cases, so too can the affected age (1). In terms of gender, SMAS has been shown to affect females more than males with a ratio of 3:2 (1,3).
In terms of clinical presentation, signs/symptoms of SMAS are often vague and/or non-specific and can be either acute or chronic. Symptoms can include nausea, vomiting, epi-gastric pain, abdominal distention, weight loss, early satiety, post-prandial discomfort. Of note, both the epigastric pain and post-prandial discomfort are likely to be position dependent where-in a supine position aggravates these symptoms while a left lateral decubitus position alleviates these symptoms. SMAS can have an acute or chronic presentation, presenting with obstructive features including nausea/vomiting, poor oral intake, gastric dilation, and weight loss. Since SMAS is highly variable in its presentation, it can easily be overlooked often leading to delays in diagnosis with potentially fatal complications. A high index of clinical suspicion is needed to ensure proper identification/diagnosis (1-4). Gastrointestinal mucosal injury/damage has been reported as a common complication with a reported rate of 25-59%, often secondary to several mechanisms (e.g., retained or refluxed peptic and/or bile acid, increased intraluminal pressure) (1,3). If not treated in a proper timeframe or manner, this may lead to sequalae such as pneumoperitoneum, emphysema, portal venous gas, or necrosis. Less dire complications such as elevations in pancreatic enzymes and/or acute pancreatitis can also occur (1).
A diagnosis of SMAS consists of both clinical symptomatology and associated imaging findings of duodenal obstruction. Multiple modalities can be utilized to assess for SMAS (e.g., CT, US, MRI, EUS); however, the most beneficial modality would be CT imaging as it can not only confirm SMAS in multiple views (Figure 1), but also provide supporting data such as SMA-AA angle/distance and/or identify the aforementioned complications of SMAS. On CT imaging, the standard SMA-AA angle, distance typically ranges from 38-65 degrees and 10-33 mm, respectively (1,3). Unal et al (2005) determined that the cutoff values of 22 degrees (SMA-AA angle) and 8 mm (SMA-AA distance) for SMAS had 42.8% sensitivity/ 100% specificity and 100% sensitivity/100% specificity–respectively. Abdominal US has been shown to be an effective modality in diagnosing SMAS. In recent literature, the use of EUS has become more commonplace as it can not only confirm duodenal compression but also now measure both SMA-AA angle and distance (1).
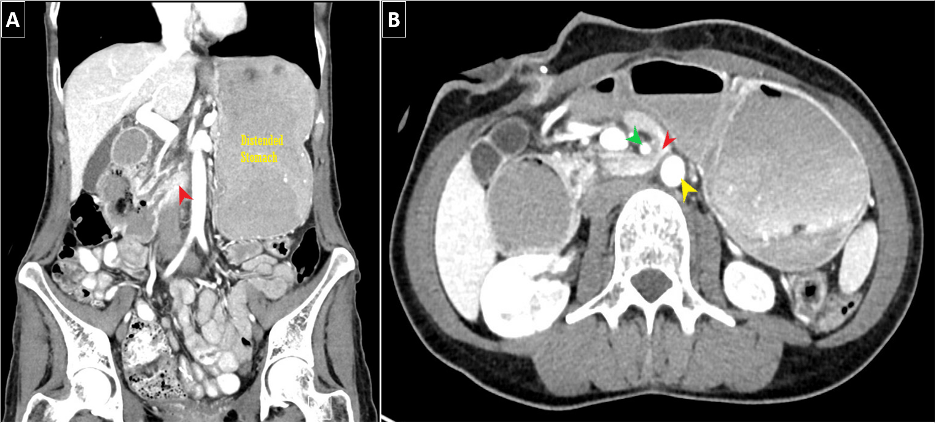
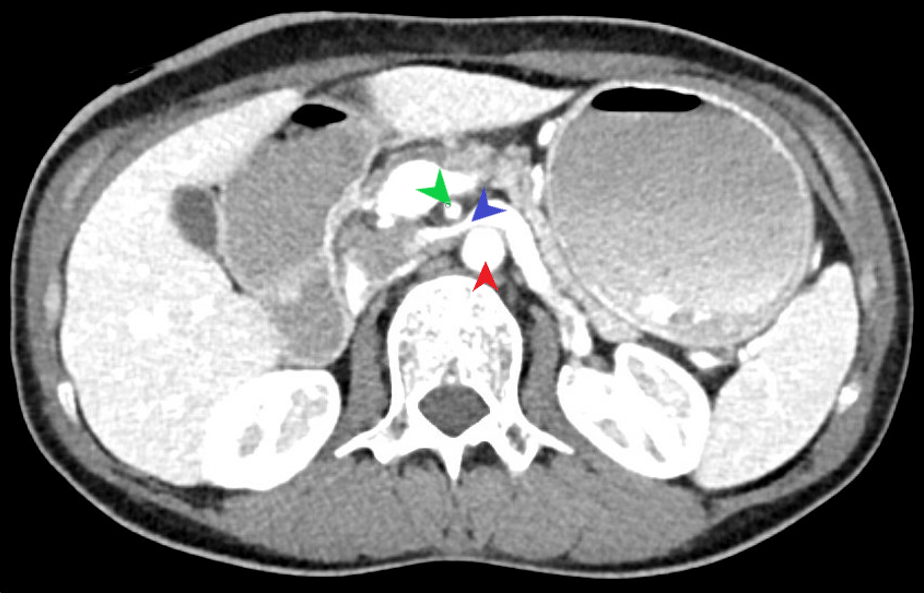
It is worthwhile to mention that SMAS can, at times, present alongside additional vascular compression syndromes. Most commonly, this being nutcracker syndrome (left renal vein compression between the SMA and AA) based on the anatomical location (Figure 2). Another example would be celiac axis compression syndrome which occurs due to compression of the celiac artery by the median arcuate ligament (1).
Besides the initial clinical stabilization, fluid resuscitation and electrolyte repletion, the treatment of SMAS relies chiefly on nutritional support. Often patients are not able to tolerate oral diet, so alternate methods of delivery of nutrition into the gut are utilized which may include placement of a naso-jejunal feeding tube. Conservative approaches using patient positioning (e.g. left lateral decubitus) have been suggested, however, have limited clinical utility and often do not translate into clinical benefit. Unfortunately, many will fail conservative therapy and require more aggressive therapy along the lines of surgical or other alternative invasive intervention (1-4).
Previously, traditional open bypass surgery was done but with the advent of minimally invasive techniques, laparoscopic duodenojejunostomy is the current preferred procedure for SMAS due to its high success rate and improved overall outcomes (3,4). In the recent literature, endoscopic ultrasound-guided (EUS) gastroenterostomy or more specifically EUS gastrojejunostomy has been done for SMAS treatment with positive results–showcasing the ever-expanding application of EUS based procedures (1).
In summary, prompt and accurate diagnosis remains a challenge owing to the non-specific nature of this condition, thus requiring a high index of clinical suspicion. Abdominal cross-sectional imaging remains the gold standard for diagnosis currently. Due to advancement in minimally invasive techniques, endoscopists have an even greater degree of impact in management of patients with this syndrome and continue to serve as a vital part of the care management team.
References:
Oka A, Awoniyi M, Hasegawa N, et al. Superior mesenteric artery syndrome: Diagnosis and management. World J Clin Cases. 2023;11(15):3369-3384.
Sinagra E, Raimondo D, Albano D, et al. Superior Mesenteric Artery Syndrome: Clinical, Endoscopic, and Radiological Findings. Gastroenterol Res Pract. 2018;2018:1937416. Published 2018 Aug 27.
Van Horne N, Jackson JP. Superior Mesenteric Artery Syndrome. [Updated 2023 Jul 17]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK482209/
Sabry A, Shaalan R, Kahlin C, Elhoofy A. Superior Mesenteric Artery Syndrome Managed with Laparoscopic Duodenojejunostomy. Minim Invasive Surg. 2022;2022:4607440. Published 2022 Aug 3.
Unal B, Aktaş A, Kemal G, et al. Superior mesenteric artery syndrome: CT and ultrasonography findings. Diagn Interv Radiol. 2005;11(2):90-95.

